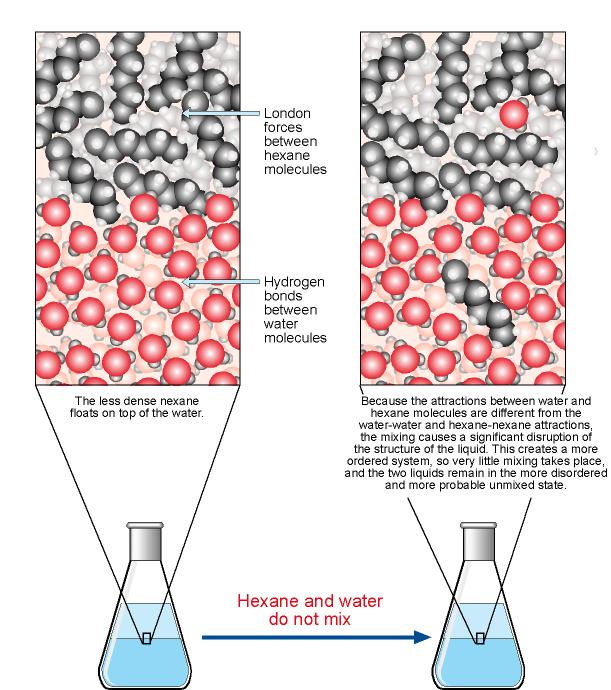
Hexane and Water Do Not Form a Solution
Water do not mix is quite distinct from the reason many other substance do not mix. Thus these elements are not being removed from the alloy being sintered.
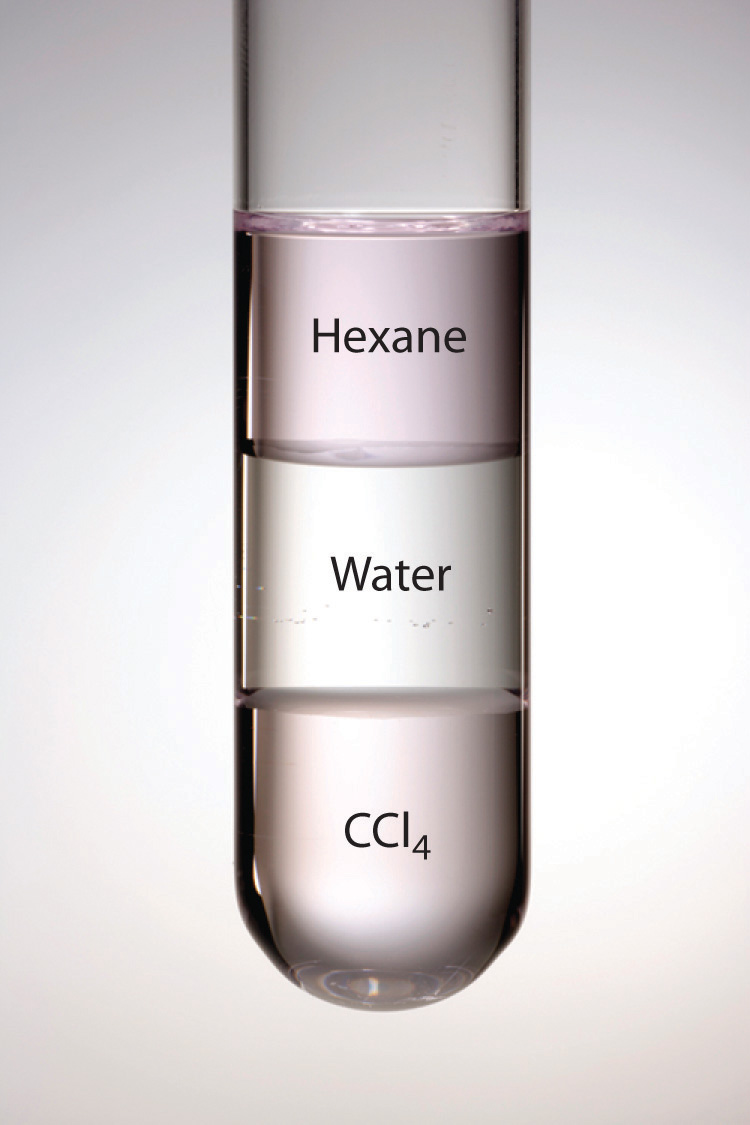
Why Can T Hexane Dissolve In Water Socratic
The reasons why hexane and water do not mix are complex but.

. The mode of action of this drug has not been clearly identified but may be related to its sedative properties. Capsule may be opened and contents dissolved in water yogurt or orange juice. The molality of an.
It is believed that the water molecules adjust to compensate for the loss of some hydrogen bonds. Where X 1 and X 2 are the respective mole fractions in solution and p v1 and p v2 are the vapor pressures of the two metals. C 6 H 6.
Furthermore which will not react with KMnO4. C 6 H 14 benzene. Again our modified law is upheld.
Two TLCs should be run as done previously. Chloroform and methylene chloride are denser than water while most other organic solvents are not as dense as water. The SI unit for molality is molkg or moles solute per kg of solvent.
Emulsions and colloids are examples of immiscible phase pair combinations that do not physically separate. The reason I. Therefore the organic layer could be above or below the aqueous layer depending on the organic solvent used.
Aqueous potassium permanganate reacts with cyclohexene to form a variety of products including cis-l2-cyclohexane- diol. To do this you need the molecular weights. White Mineral OilAlso called paraffin oil or mineral oil.
Increased temperature usually increases the solubility of solids in liquids. C 5 H 12 hexane. What is the mole fraction of hexane.
In separate labeled containers obtain 15 mL hexane 15 mL of 70 hexane-30 acetone solution 15 mL acetone and 15 mL of 80 acetone-20 methanol solution. The intermolecular force of hydrogen bond in water molecule itself is stronger than the induced dipole attraction between the polar bond in water and the nonpolar bond gas oxygen. In fact according to Smith et al if the vapor.
A salt water solution. All the ions and proteins in a cell are dissolved in water within the cell. 3 Then divide the moles of hexane by the total.
When a purple solution of the oxidizing agent KMnO4 is added to an alkene the alkene is oxidized to a diol and the. Table salt readily dissolves in water to form a solution. A number of substances and classes of compounds associated with condensation or oxidation-reduction reactions.
You will also see that the hydrophobic effect is part of a family of processes called entropy driven ordering and that the hydrophobic effect has nothing to do with bonds between hydrophobic molecules. 1 part ethyl acetate. Hexane Reagent Grade less than 100 mg kg to 200 mg kg water WarningFlammable.
Methocarbamol does not directly relax tense skeletal. 5 hrs 10-13 hours. On the side they added some babble to make up the same amount of lines which they are not showing with their new look but the description on the actual bottle does not list cold pressed or hexane free and does not match their advertisement.
Benzoic acid is only sparingly soluble in cold acidic water. Since the column does not have a stopcock to stop the flow of solvent during the procedure all the solvents must be at your workspace before starting the process. The solubility at 50 C is 244 g100 mL of water.
Once the solution is acidic. This molecule is almost. Methanol and ethanol are not useful extraction solvents because they are miscible with water and will not form a separate layer.
The yield of the latter product is markedly enhanced by turbulent stirring 1. If the masses of the salt and of the water are known the molality can be determined. Please note that methanol dissolves but does not form ions in solution.
A solution with a molality of 1 molkg is often described as 1 molal or 1 m However following the SI system of units the National. New attractions between hexane and water molecules do form but because the new attractions are very different from the attractions that are broken they introduce significant changes in the structure of the water. CH 3 CH 2 6 CH 2 OH - octanol.
If you are not sure. A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. 2 When you have the moles of each add them together.
Octanol a mostly nonpolar molecule dissolves in hexane but formed a separate layer in water. Also label six test tubes. In the second plate you will be able to see the purity of the naphthalene fraction.
Notice that oxygen does not chemically react with water since oxygen is a non-polar gas. 1 You need to determine the moles of pentane hexane and benzene. Here are the formulas.
Visualize the spots using the short-wave UV lamp. 84 one can see that the metals with the lower vapor pressures will have less effect on the composition of the vapor. Dried over molecular sieve.
The injectable form of methocarbamol is indicated as an adjunct to rest physical therapy and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions. Phases do not need to macroscopically separate spontaneously. The first plate will provide information on the identity and purity of your product.
The new version does not state that and they very carefully do not show the bottle from any other side than the front. This should sound a bit strange to you right now but I am sure that at the end of the class it. Molecular Sieve 5Å8 to 12 mesh.
For example the solubility of glucose at 25 C is 91 g100 mL of water. Left to equilibration many compositions will form a uniform single phase but depending on the temperature and pressure even a single substance may separate into two or. We can use our tentative law to predict whether the following substances are soluble in water or hexane.
If we add 100 g of glucose to 100 mL water at 25 C 91 g dissolve. A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution.

Solved Question 1 Compare The Action Of Detergent On Hexane Chegg Com

0 Response to "Hexane and Water Do Not Form a Solution"
Post a Comment